Comparative Evaluation of Blood Gas and Biochemistry Analyzers in Lactate Measurement in Pediatric Patient Groups
DOI:
https://doi.org/10.14744/ajh.91Keywords:
blood gas analysis, inter‐device variability, lactic acidAbstract
Objective:
Lactate plays an important role in clinical decision-making processes as an early biochemical marker of tissue hypoxia and hypoperfusion. The aim of this study is to compare lactate measurements obtained from blood gas and biochemistry analyzers in pediatric patients and to evaluate the clinical concordance between the methods.
Methods:
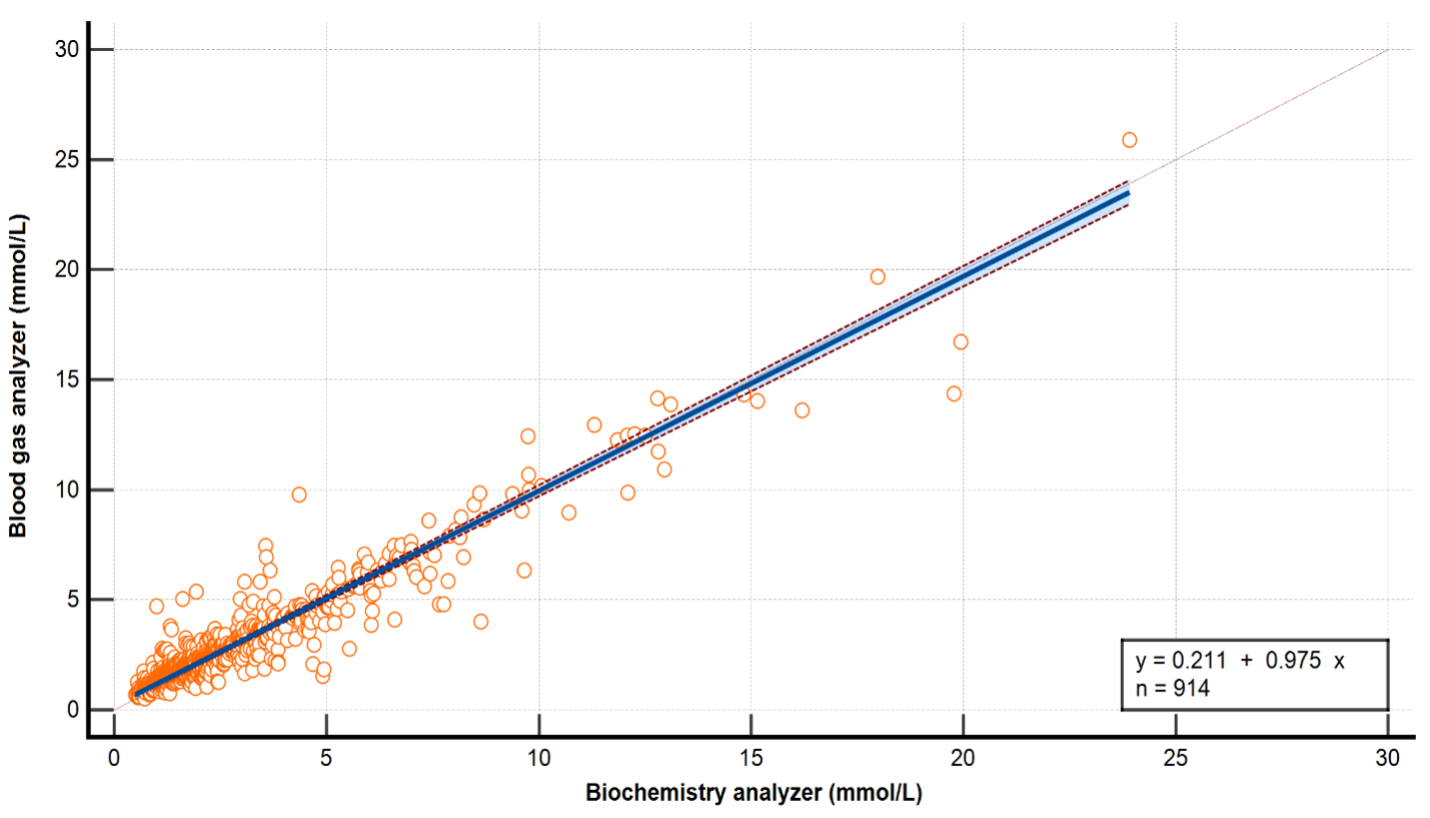
Pediatric cases with lactate measurements performed simultaneously on blood gas and biochemistry analyzers at the Ankara Etlik City Hospital Biochemistry Laboratory between January 2023 and August 2025 were retrospectively reviewed. A total of 914 paired lactate measurements were included in the analysis. Measurements were evaluated using Pearson correlation, Bland–Altman analysis, Passing–Bablok regression, and Cohen's kappa coefficient. A total allowable error (TEa) of ±0.20 mmol/L was considered the clinical acceptance criterion.
Results:
A very strong positive correlation was found between blood gas and biochemistry measurements (r = 0.95, p < 0.0001). In the Bland–Altman analysis, the mean bias was −0.13 mmol/L (95% CI: −0.18 to −0.08), the lower limit of agreement (LoA) was −1.56 mmol/L, and the upper LoA was 1.31 mmol/L; 95.1% of the results fell within these limits. The Passing–Bablok regression equation was y = 0.211 + 0.975·x (intercept 0.211, 95% CI: 0.168–0.251; slope 0.975, 95% CI: 0.954–0.996), and no deviation from linearity was observed (CUSUM p = 0.97). In the categorical analysis, the fit was good, with Cohen's kappa found to be 0.73 (95% CI: 0.70–0.76), and the misclassification rate remained below 10%.
Conclusion:
This study demonstrated that blood gas and biochemistry analyzers showed a high degree of agreement in lactate measurements in the pediatric patient group. The advantage of blood gas devices in providing rapid results is an important support element for clinicians, particularly in intensive care and emergency departments. Although minor differences were observed between the methods at low and high concentrations, it was determined that these differences were not significant enough to influence clinical decisions.
References
Ramanathan R, Parrish DW, Hartwich JE, Haynes JH. Utility of admission serum lactate in pediatric trauma. J Pediatr Surg. 2015 Apr;50(4):598-603.
Vincent JL, Quintairos E Silva A, Couto L Jr, Taccone FS. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care. 2016 Aug 13;20(1):257.
Scott HF, Donoghue AJ, Gaieski DF, Marchese RF, Mistry RD. The utility of early lactate testing in undifferentiated pediatric systemic inflammatory response syndrome. Acad Emerg Med. 2012 Nov;19(11):1276-80.
Kraut JA, Madias NE. Lactic acidosis. N Engl J Med. 2014 Dec 11;371(24):2309-19.
Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care. 2013 May 10;3(1):12.
Ismail F, Mackay WG, Kerry A, Staines H, Rooney KD. The accuracy and timeliness of a Point Of Care lactate measurement in patients with Sepsis. Scand J Trauma Resusc Emerg Med. 2015 Sep 17;23:68. doi: 10.1186/s13049-015-0151-x. Erratum in: Scand J Trauma Resusc Emerg Med. 2016 Mar 31;24:41.
Jansen TC, van Bommel J, Bakker J. Blood lactate monitoring in critically ill patients: a systematic health technology assessment. Crit Care Med. 2009 Oct;37(10):2827-39.
Measurement Procedure Comparison and Bias Estimation Using Patient Samples; Approved Guideline. 3rd ed. CLSI document EP09-A3. Wayne (PA): Clinical and Laboratory Standards Institute; 2013.
Data Innovations. Total Allowable Error Table [Internet]. Eau Claire, WI: Data Innovations; 2025 Jun 30 [cited 2025 Sep 20]. Analyte: Lactate, Serum ±0.2 mmol/L (WSLH). Available from: https://www.datainnovations.com/allowable-total-error-table/
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159-74. PMID: 843571.
Karon BS, Scott R, Burritt MF, Santrach PJ. Comparison of lactate values between point-of-care and central laboratory analyzers. Am J Clin Pathol. 2007 Jul;128(1):168-71.
Marija K, Bernhard KF, Beatrice LK. Blood-gas vs. Central-Laboratory analyzers: interchangeability and reference intervals for sodium, potassium, glucose, lactate and hemoglobin. Heliyon. 2021 Oct 30;7(11):e08302.
Jeong TD, Kim SK. Performance of the New RAPIDPoint 500e Blood Gas Analyzer and its Comparison with Central Laboratory Biochemistry Analyzer. Clin Lab. 2022 Sep 1;68(9).
Mohammed-Ali Z, Bagherpoor S, Diker P, Hoang T, Vidovic I, Cursio C, et al. Performance evaluation of all analytes on the epoc® Blood Analysis System for use in hospital surgical and intensive care units. Pract Lab Med. 2020 Nov 25;22:e00190.
Downloads
Published
How to Cite
Issue
Section
Categories
License
Copyright (c) 2025 Aziz Sener, Serhat Takil, Arzu Kosem

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.

